Determining the Concentration of a Solution: Beer’s Law
Purpose
To determine the wavelength
(color) of maximum absorbance for a copper (II) sulfate solution.
To examine the relationship between the absorbance and concentration of a
copper (II) sulfate solution.
To determine the concentration of an unknown copper (II) sulfate solution.
Introduction
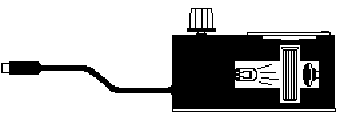
You will be using the
Colorimeter shown in Figure 1. In this device, light from an LED light
source will pass through the solution and strike a photocell. The CuSO4
solution used in this experiment has a deep blue color. A higher concentration
of a colored solution will absorb more light and transmit less light than a
solution of lower concentration. The colorimeter will interpret the light
received by the photocell and express it both as an absorbance and a percent
transmittance value.
 |
 |
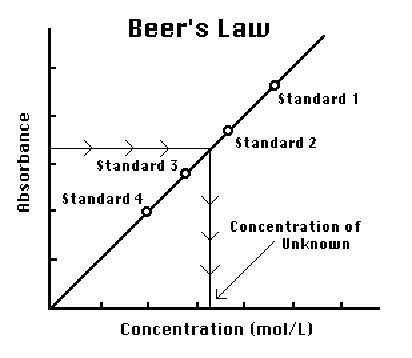
| Figure 1 | Figure 2 |
You will prepare five copper (II) sulfate solutions of known concentration (standard solutions). Each solution will be transferred to a small, rectangular cuvette that is placed into the colorimeter. The absorbance of each solution will be measured and recorded. When a graph of absorbance vs. concentration is plotted for the standard solutions, a direct relationship should result, as shown in Figure 2. This direct relationship is known as Beer's Law, and is shown by the equation
A = εcl
where A is the absorbance measured with a colorimeter, ε is the molar absorptivity, a constant for the particular solute you are analyzing, c is the molar concentration of the solute, and l is path length, the distance light travels through the cuvette (usually 1 cm). For a single solute, absorbance and concentration are directly proportional if the path length is constant. When a linear trendline analysis is performed on a graph of absorbance vs. concentration, the slope is equal to the molar absorptivity, ε, if the path length is 1 cm.
The concentration of an unknown CuSO4 solution will then be determined by measuring its absorbance with the colorimeter and using the molar absorptivity from your trendline equation to determine its concentration.
| Materials | |
| computer with Logger Pro 3.2 and Interface | 30 mL of 0.50 M CuSO4 |
| Vernier Colorimeter | 5 mL of an unknown CuSO4 solution |
| two 1-mL, two 2-mL pipets (or a micropipet) | pipet pump(s) or bulb(s) |
| 1-cm square plastic colorimeter cuvette | KimWipes® or similar laboratory wipes |
| stirring rod | two 100-mL beakers |
| five small, labeled test tubes | test tube rack |
Procedure
|
Table 1 |
|
| Test Tube | CuSO4 concentration (M) |
| 1 | 0.10 |
| 2 | 0.20 |
| 3 | 0.30 |
| 4 | 0.40 |
| 5 | 0.50 |
| HANDLING CUVETTES: Examine your cuvette to ensure the clear sides are free from scratches. Cuvettes should be wiped clean and dry on the outside with a KimWipe before each measurement. Do not use a paper towel! Handle the cuvettes near the top of the ribbed sides. Solutions should be free of bubbles. If you are refilling a cuvette with a different solution, a small amount of the new solution should be used to rinse the cuvette before filling. Always align the reference mark on the cuvette with the reference mark on the slot of the colorimeter. To avoid inconsistencies from different cuvettes, only one cuvette should be used for the entire experiment. |
| CALIBRATION of SQUARE COLORIMETERS: Click the icon on the toolbar used to calibrate the probes. (Alternatively, select "Calibrate" from the "Experiments" menu.) Place the blank cuvette in the cuvette slot of the colorimeter and close the lid. Turn the wavelength knob of the colorimeter to the 0% T position. In this position, the light source is turned off, so no light is received by the photocell. Type "0" in the % edit box. When the voltage stabilizes, click "Keep." Turn the knob of the colorimeter to the desired wavelength. In this position, the sample transmits 100% of the light. Type "100" in the % edit box. When the voltage stabilizes, click "Keep." Then click "OK." |
| CALIBRATION of ROUNDED COLORIMETERS: Use the arrow buttons to select the desired wavelength. With the blank cuvette correctly positioned in the colorimeter, press the blue "Cal" button on top of the colorimeter. When the red light stops blinking, the colorimeter is calibrated and may be used. |
| FINDING THE WAVELENGTH OF MAXIMUM ABSORBANCE (λmax): Before making any absorbance measurements with the colorimeter, the wavelength (color) of maximum absorbance must be determined. This may be done by first calibrating the colorimeter at one of the available wavelengths as shown above. Then, place a cuvette containing the most concentrated sample into the colorimeter and measure the absorbance. Repeat this calibration and measurement procedure for the remaining wavelengths on your colorimeter. The wavelength that yields the greatest absorbance reading for this sample is called λmax. Re-calibrate your colorimeter at this wavelength if necessary. This is the wavelength you should use for all of your future measurements. No further calibrations should be necessary. |
Lab Report
Written by Lance S. Lund, Anoka-Ramsey Community College. Portions of this lab courtesy of Kirk Boraas, Minneapolis Community and Technical College, and Vernier Software, "Chemistry With Computers". Updated December 05, 2011.