Determination
of an Equilibrium Constant
Introduction
A state of chemical equilibrium exists when the rate of the forward reaction is equal to the rate of the reverse reaction. Once equilibrium has established itself, the amounts of products and reactants are constant. Furthermore, if one of the product or reactant concentrations can be measured, it can be used to determine the remaining concentrations and the equilibrium constant.
In this experiment, we will begin by combining aqueous solutions of iron(III) ion (Fe3+) and thiocyanate ion (SCN-). The reaction that occurs produces the thiocyanoiron(III) complex ion responsible for the equilibrium mixture's deep red color.
![]()
iron(III) thiocyanate thiocyanoiron(III)
The final state of the equilibrium (i.e., the final concentrations of products and reactants) depends on the relative amounts of reactants before the reaction occurs. However, regardless of the initial concentrations, the final equilibrium concentrations must satisfy the following relationship:
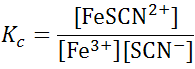
where the bracketed terms are molar equilibrium concentrations of the different species, and Kc is the temperature-dependent equilibrium constant.
In today's experiment, we will study the chemical equilibrium described above by varying the initial concentrations of each reactant. After equilibrium is achieved, the colorimeter will be used to determine the concentration of thiocyanoiron(III) complex ion. This is then used to determine the concentrations of iron(III) ion and thiocyanate ion . These concentrations are then substituted into the expression for Kc to check and determine whether the equilibrium constant is really constant from one case to the next.
The molar absorptivity, ε, will be used to convert measured absorbances (via the colorimeter) to FeSCN2+ concentrations. The molar absorptivity of FeSCN2+ will be obtained by using a standard solution that contains an initial 100x excess of Fe3+ ion in comparison to SCN-. According to Le Châtelier's principle, this high concentration shifts the reaction far to the right in favor of the product, using up approximately 100% of the SCN- ions. Consequently, the [FeSCN2+] in the equilibrium mixture is approximately equal to the original [SCN-]. The [FeSCN2+] for each of the equilibrium mixtures (vials) may then be determined from their absorbances and the molar absorptivity.
Knowing the equilibrium concentration of the product FeSCN2+ species, it is possible to determine the equilibrium concentrations of reactants. First calculate the concentration of each original reactant before equilibrium was achieved using initial volume and concentration information. Next calculate the concentration of FeSCN2+ formed. Since products and reactants are in a 1:1 mole ratio and any product formed reduces the amount of reactant remaining in the reaction mixture, the concentration of remaining reactant is determined by:
equilibrium concentration reactant = initial concentration reactant - equilibrium concentration product
Calculate the concentration of each reactant by dividing the number of moles of reactant by the mixture's total volume. Use these concentrations to calculate the equilibrium constant for each of the mixtures.
The colorimeter used in today's experiment will be set to a wavelength of 470 nm. This wavelength is used since it is readily absorbed by the FeSCN2+ complex ion. As the concentration of the sample increases, the amount of light that passes through the sample decreases, and the absorbance increases.
Procedure
|
Vial Number |
1 |
2 |
3 | 4 | 5 | 6 |
|
Fe(NO3)3 (mL) |
1.0 |
2.0 |
4.0 | 5.0 | 6.0 | 8.0 |
| KSCN (mL) |
9.0 |
8.0 |
6.0 |
5.0 | 4.0 | 2.0 |
Data Analysis and Questions
Data Table
Use Excel to create a
data table that includes six columns—one
for each of the six vials. The following information should be included as rows
for each trial :
Also include the absorbance for the standard solution and the average values of Kc at the bottom of the table.
Lab Report
You may choose to submit an individual or
group report for this lab. Follow the guidelines for
Laboratory Reports located at
http://webs.anokaramsey.edu/chemistry/Chem1062.
For this lab report, you will need to include a
title, abstract, procedure, results,
discussion, and references. You may use the above questions to
guide your discussion, but the discussion should be more than
just answering the questions and should flow
logically as you discuss the lab and the results.
Follow your instructor’s directions for submitting this lab report. Remember to name the file as specified (Lastname_Equilibrium or Lastname1_LastName2_Equilibrium). If you are emailing your report, use the subject line “Chem 1062: Equilibrium”. Also, embed the Excel table in the Word document so that the professor may view the formulas in each cell. If you submit a paper copy of your report, include one set of sample calculations for one of the trials, either handwritten or typed. If you worked in pairs and are submitting this assignment on an individual basis, please underline your own name and include your lab partner’s name on the assignment.
Portions of this lab courtesy of Kirk Boraas, Minneapolis Community and Technical College, and Vernier Software, "Chemistry With Computers". Edited by Lance S. Lund, Anoka-Ramsey Community College. Updated April, 2011.