Determining the
Concentration of a Solution: Beer’s Law
Purpose
To determine the wavelength
(color) of maximum absorbance for a copper (II) sulfate solution.
To examine the relationship between the absorbance and concentration of a
copper (II) sulfate solution.
To determine the concentration of an unknown copper (II) sulfate solution.
Introduction
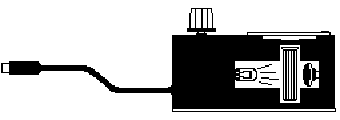
You will be using the
Colorimeter shown in Figure 1. In this device, light from an LED light
source will pass through the solution and strike a photocell. The CuSO4
solution used in this experiment has a deep blue color. A higher concentration
of a colored solution will absorb more light and transmit less light than a
solution of lower concentration. The colorimeter will interpret the light
received by the photocell and express it both as an absorbance and a percent
transmittance value.
 |
 |
| Figure
1 |
Figure
2 |
You will prepare five copper (II)
sulfate solutions of known concentration (standard solutions). Each solution
will be transferred to a small, rectangular cuvette that is placed into the
colorimeter. The absorbance of each solution will be measured and recorded. When
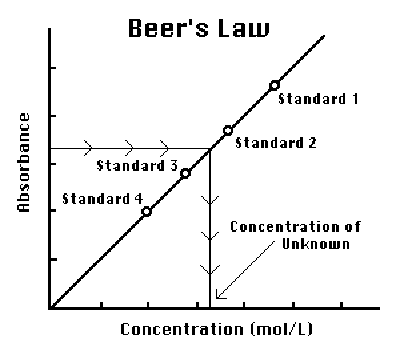
a graph of absorbance vs. concentration is plotted for the standard solutions, a
direct relationship should result, as shown in Figure 2. The direct
relationship between absorbance and concentration for a solution is known as
Beer’s Law. The concentration of an unknown CuSO4 solution
will then be determined by measuring its absorbance with the colorimeter and
using the equation of the best-fit line to determine its concentration.
|
Materials |
|
| computer
with Logger Pro 3.2 and Interface |
30 mL of 0.50 M
CuSO4 |
| Vernier Colorimeter |
5 mL of an unknown
CuSO4 solution |
| two 1-mL, two 2-mL pipets
(or a micropipet) |
pipet pump(s) or
bulb(s) |
| a set of matched
cuvettes |
Kimwipes® |
| stirring rod |
two 100-mL beakers |
| five small, labeled
test tubes |
test tube rack |
Procedure
- Obtain and wear goggles! CAUTION:
Be careful not to ingest any CuSO4 solution or spill any on
your skin. Inform the professor immediately in the event of an accident.
- Pour about
15 mL of 0.50 M CuSO4 stock solution into a 50-mL beaker.
Pour about 15 mL of deionized water into a different 50-mL beaker.
- Into five clean, dry, and labeled
test tubes, prepare 5.00 mL of the following solutions by dilution, using the 0.50 M
CuSO4 and the pipets available to you in the lab. (Note: If
you are unsure of how to read the pipets, please ask. The most common
mistake in this lab involves measurements with the pipets.) Prepare a
table on Sheet 1 of an Excel spreadsheet that shows the
amount of 0.50 M CuSO4 and amount of water used in each
test tube. Stopper and mix the contents of each test tube thoroughly.
|
Table 1 |
| Test
Tube |
CuSO4
concentration (M) |
| 1 |
0.10 |
| 2 |
0.20 |
| 3 |
0.30 |
| 4 |
0.40 |
| 5 |
0.50 |
- Save your spreadsheet
using a filename convention of: Lastname1_Lastname2_Lab11.
- Connect the colorimeter to the
LabPro® interface, using an adapter, if necessary. Next,
open the Logger Pro application from the desktop or the Start menu. From
within Logger Pro, open the "Probes & Sensors" folder,
then select the "Colorimeter" folder,
and finally the "Absorbance-Conc" file.
- You are
now ready to use the colorimeter. Prepare a blank by filling a
cuvette 3/4 full with deionized water. Place each of the of the solutions
prepared in Step 3 above into separate cuvettes. The cuvettes should be
filled approximately 3/4 full. Calibrate the colorimeter at lmax
for the copper (II) sulfate solution.
| HANDLING
CUVETTES: Cuvettes should be
wiped clean and dry on the outside with a Kimwipe®. Do not use a
paper towel! Handle the cuvettes near the top of the ribbed sides. Solutions should be free of
bubbles. If a cuvette is not dry on the inside, a small amount of the
solution to be used should be used to rinse the cuvette before
filling. Always align the reference mark on the cuvette with the
reference mark on the slot of the colorimeter. |
| FINDING
THE WAVELENGTH OF MAXIMUM ABSORBANCE (lmax):
Before making any measurements with the colorimeter, the wavelength
(color) of maximum absorbance must be determined. This may be done by
following the calibration procedure below for each of the wavelengths
available on the colorimeter. Once the colorimeter is calibrated at a
particular wavelength, place a cuvette containing the most concentrated
sample into the colorimeter. The wavelength that yields the greatest
absorbance reading for this sample is called lmax
and is the wavelength you should use for all of your measurements. |
|
CALIBRATION: Click the
"LabPro" icon on the toolbar.
Then click on the "CH1" icon and select
"calibrate". Then click on the "Calibrate Now" button. Place the
blank cuvette in the cuvette slot of the colorimeter and close the lid.
Turn the wavelength knob of the colorimeter to the 0%T position. In
this position, the light source is turned off, so no light is received by
the photocell. Type "0" in the
Reading 1 Value box. When the voltage
stabilizes, click "Keep". Turn the knob of the colorimeter to the desired
wavelength. In this position, the sample transmits 100% of the light. Type
"100" in the
Reading 2 Value box. When the voltage
stabilizes, click "Keep", then click "Done". |
- Once,
you have determined lmax
and calibrated the colorimeter at lmax, measure the absorbance
for each of the solutions in Table 1, as well as the absorbance of
the deionized water blank, by placing them into the colorimeter,
aligning the reference marks, closing the lid, and measuring their
absorbances. Record the results on Sheet 2 of your spreadsheet that
shows the molarity of CuSO4 in each solution and each of their
absorbances. Make sure to include the value of the wavelength at which you
are making your measurements.
- Using Excel, prepare a graph
that plots absorbance vs. concentration. Obtain the equation of the best-fit
line, setting the y-intercept to zero. (Why
should the y-intercept be set to zero?)
- Fill a cuvette about 3/4 full with
your unknown sample of CuSO4. Record your unknown number.
Measure the absorbance of the unknown as before. If the
absorbance is greater than any of the measurements used to prepare your
trendline, dilute the solution and measure the absorbance again. Take note of
the volumes used in your dilution.
- Use the equation of your
best-fit line to determine the concentration of the
unknown solution (use a formula
inside a spreadsheet cell). If you
diluted your unknown, calculate the concentration of the
undiluted solution and report the result. Clearly label the your unknown number and its
concentration.
- Discard the solutions as directed by
your instructor. Clean your cuvettes. Do not use test tube brushes or paper
towels, as they may scratch the surface of the cuvettes. Use Kimewipes® to
dry the cuvettes.
Assignment
- This will be a short
report. In this report, you will need to include the title,
experimental
details, results (the table and graph you made above which can be embedded
into a Word document), discussion, and references.
- As a part of the
discussion section, you will need to include the following:
- describe
the
preparation of 500.0 mL of 0.50 M CuSO4,
using
solid CuSO4 and water,
- using
your trendline equation and
Beer's Law,
calculate the absorbance for 0.25 M CuSO4
at lmax,
- any errors that
occurred or other observations made.
- Follow your instructor's
directions for submitting this lab report. If you submit electronically, be
sure to follow the filename convention mentioned earlier. If emailing, use
"Chem 1061: Beer's Law Law" as the subject line. If
you turn in a paper copy or do NOT embed your Excel file into your Word
document, you will need to provide a sample calculation for determining the
concentration of your unknown solution.
Written by Lance S. Lund, Anoka-Ramsey
Community College. Portions of this lab courtesy of Kirk Boraas, Minneapolis Community and
Technical College, and Vernier Software,
"Chemistry With Computers". Updated
October 22, 2007.



