Solubility of a Salt
Introduction
Solubility is defined to be the maximum amount of solute that will dissolve
in a given amount of solvent at a specified temperature. Typically, solubilities
are recorded as grams of solute per 100 g of solvent. For many solutes,
increasing the temperature increases the solubility, that is, more of the
solute dissolves in the same amount of solvent. In this experiment, you will
characterize the temperature behavior of a solution made up of KNO3
dissolved in water.
As noted below, you will begin by combining different amounts of KNO3
in similar amounts of water. Most of these systems will require heating before
the solute will completely dissolve. After the solid completely dissolves, the
solution is constantly stirred and allowed to cool. At some point, the
temperature will be low enough for the solution to become saturated.
This point is indicated by the formation of small crystals (that look like
snowflakes) within the solution system.
Noting the temperature when crystals form and knowing the amounts of solute
and solvent permit us to construct a solubility graph. This is a graph of
solubility (y axis) vs. temperature (x axis). After plotting the
temperature and solubility for all of your solutions, you will connect the dots
with a smooth line used to interpolate solubilities between the dots.
Lastly, you will be given an unknown solution. After experimentally measuring
the temperature corresponding to the appearance of crystals, you will use your
previously constructed solubility graph to determine the unknown's
concentration.
Apparatus:
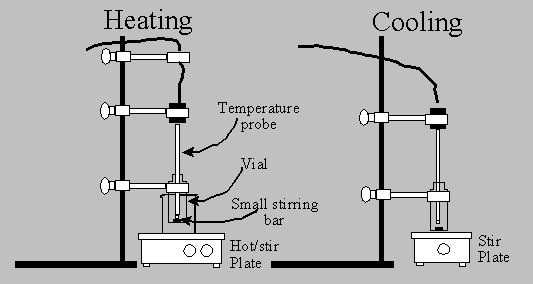
Procedure
1.
Construct the apparatus shown above. Note that
initially, heating and stirring are accomplished by using the hot/stir plate.
As the solution is cooled, a second cool hotplate (or stir plate) is used to
stir the mixture.
IMPORTANT: Safety goggles must be worn for this experiment!
2.
Obtain six vials from the lab instructor and
label appropriately. Mass each vial (cap on) and record each respective mass.
On a piece of weighing paper, measure out the amount of KNO3 given
by the table below. Transfer the solid to its vial and re-mass to determine the
amount of solid actually transferred to the vial. Repeat this procedure for all
six vials.
|
Vial # |
Approximate KNO3 mass (g) |
|
1 |
~3.0 g |
|
2 |
~2.5 g |
|
3 |
~2.0 g |
|
4 |
~1.5 g |
|
5 |
~1.0 g |
|
6 |
~0.5 g |
- Into each vial, pipette 2.00 mL of deionized water.
Re-mass each vial to determine the amount of water actually transferred to
the vial.
- Connect a stainless steel temperature probe to the LabPro
interface and open the Vernier LoggerPro application. From the
"Insert" menu, select a digital meter. There is no need to
calibrate the temperature probe, if the stainless steel probe is used.
IMPORTANT: Position all wires away from the hot plate!!! Always be watchful of the wires whenever you reposition the apparatus!
- Remove the cap from vial #1 and insert a small magnetic
stir bar. Use clamps to support the vial in the hot water bath (a 50-mL or
100-mL beaker). Note that the bottom of the vial must be close to the
bottom of the water bath for the magnetic stirrer to work.
- Continue heating the solution until all of the solid
has dissolved. Do not leave the vial in the water bath any longer than
what is required to completely dissolve the solid.
- When the KNO3 has dissolved, move the
apparatus to a cold stir plate and continue stirring. Insert the
temperature probe into the vial, making sure that the probe is clean and
dry. Allow the vial to cool slowly (it will be necessary to construct a
cold water bath in a small beaker to accelerate the process in some
cases).
- At some point, crystals will form and gradually build
in intensity as the solution cools (snow storm). Record the temperature on
the digital meter at the instant crystals form. If the appearance of
crystals is very sudden, supercooling of the solution may have occurred
and it will be necessary to repeat the trial reheat the apparatus and
repeat the cooling process (always stirring) as necessary to obtain a
good, reproducible temperature measurement. You may need to use cool water
or an ice bath to obtain low enough temperatures to attain crystal
formation. Don't cool too quickly or you may miss the appearance of
crystals.
- Empty the vial in the appropriate waste container.
Repeat steps 5-8 with each of the numbered vials.
- Obtain an unknown KNO3 solution from the
laboratory instructor (record the unknown number and include this in your
report). Heat the solution until the solid completely dissolves. Cool the
solution and record the temperature when crystals form. Repeat as
necessary to obtain a reproducible temperature measurement.
Data Analysis and Questions
- Use Microsoft Excel to construct a data table
containing columns labeled with Vial number, T (ºC), g KNO3, g
H2O, and Solubility (gsalt/100 gsolvent)
for all six trials. Include a separate entry for the unknown solution's
identification number, its characteristic temperature, and its
concentration.
- Construct a solubility graph using the temperature and
solubility information for the first six trials. (Use the scatter plot
option, but do not connect the dots.)
- Perform a linear trendline analysis on the data. Then
try a second order polynomial trendline analysis. Note and record the
equation and correlation coefficient (R2) for each trendline.
The equation that best fits the data will have a correlation coefficient
closest to 1.
- Calculate the concentration of your unknown solution using
the equation for the trendline that best fits your data. Confirm this
result visually by checking the position of the data point on your
trendline.
- Answer the following questions:
|
A. |
Using
your graph, tell if each of these solutions would be unsaturated, saturated,
or supersaturated: |
|
|
i. |
110
g of KNO3 in 100 g of H2O at 40°C |
|
|
ii. |
60
g of KNO3 in 100 g of H2O at 70°C |
|
|
iii. |
140
g of KNO3 in 200 g of H2O at 60°C |
|
|
B. |
According
to your graph, will 50 g of KNO3 completely dissolve in 100 g of H2O
at 50°C? Explain. |
|
|
C. |
Using
your trendline equation, how many grams of KNO3 will dissolve in
100 g of H2O at 30°C? |
|
Lab Report
You may choose to submit an individual or group report for this lab.
Follow the guidelines for Laboratory Reports at http://webs.anokaramsey.edu/chemistry/Chem1062.
Please read these guidelines very carefully before beginning the report,
especially if you did not take Chemistry 1061 lab last semester. In your
report, please include a title, procedure, results, and discussion.
In the discussion, please make sure to include the identity of your
unknown and how you were able to make this determination. The answers to
question 5 should be included as an appendix at the end of the report.
Follow your instructor’s directions for submitting this lab report. Remember to name the file as specified (Lastname_Solubility or Lastname1_LastName2_Solubility). If you are emailing your report, use the subject line “Chem 1062: Solubility Lab”. If you worked in pairs and are submitting this assignment on an individual basis, please underline your own name and include your lab partner’s name on the assignment.
Lab courtesy of Vernier Software, "Chemistry With Computers"; and Kirk Boraas, Minneapolis Community and Technical College. Edited by the Anoka-Ramsey Community College Chemistry Dept. Last update was April, 2011.